|
| Appearance |
|---|
Lemon yellow sintered microcrystals


Spectral lines of sulfur |
| General properties |
|---|
| Name, symbol, number | sulfur, S, 16 |
|---|
| Pronunciation | /ˈsʌlfər/ sul-fər |
|---|
| Element category | nonmetal |
|---|
| Group, period, block | 16, 3, p |
|---|
| Standard atomic weight | 32.065(5) |
|---|
| Electron configuration | [Ne] 3s2 3p4 |
|---|
| Electrons per shell | 2, 8, 6 (Image) |
|---|
| Physical properties |
|---|
| Phase | solid |
|---|
| Density (near r.t.) | (alpha) 2.07 g·cm−3 |
|---|
| Density (near r.t.) | (beta) 1.96 g·cm−3 |
|---|
| Density (near r.t.) | (gamma) 1.92 g·cm−3 |
|---|
| Liquid density at m.p. | 1.819 g·cm−3 |
|---|
| Melting point | 388.36 K, 115.21 °C, 239.38 °F |
|---|
| Boiling point | 717.8 K, 444.6 °C, 832.3 °F |
|---|
| Critical point | 1314 K, 20.7 MPa |
|---|
| Heat of fusion | (mono) 1.727 kJ·mol−1 |
|---|
| Heat of vaporization | (mono) 45 kJ·mol−1 |
|---|
| Molar heat capacity | 22.75 J·mol−1·K−1 |
|---|
| Vapor pressure |
|---|
| P (Pa) | 1 | 10 | 100 | 1 k | 10 k | 100 k |
| at T (K) | 375 | 408 | 449 | 508 | 591 | 717 |
|
| Atomic properties |
|---|
| Oxidation states | 6, 5, 4, 3, 2, 1, -1, -2
(strongly acidic oxide) |
|---|
| Electronegativity | 2.58 (Pauling scale) |
|---|
Ionization energies
(more) | 1st: 999.6 kJ·mol−1 |
|---|
| 2nd: 2252 kJ·mol−1 |
| 3rd: 3357 kJ·mol−1 |
| Covalent radius | 105±3 pm |
|---|
| Van der Waals radius | 180 pm |
|---|
| Miscellanea |
|---|
| Crystal structure | orthorhombic |
|---|
| Magnetic ordering | diamagnetic[1] |
|---|
| Electrical resistivity | (20 °C) (amorphous)
2×1015 Ω·m |
|---|
| Thermal conductivity | (amorphous)
0.205 W·m−1·K−1 |
|---|
| Bulk modulus | 7.7 GPa |
|---|
| Mohs hardness | 2.0 |
|---|
| CAS registry number | 7704-34-9 |
|---|
| Most stable isotopes |
|---|
| Main article: Isotopes of sulfur |
|
| · r |
Sulfur (
US English) or
sulphur (
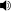 /ˈsʌlfər/ sul-fər
/ˈsʌlfər/ sul-fər;
see spelling below) is the
chemical element with
atomic number 16. In the
periodic table it is represented by the symbol
S. It is an
abundant,
multivalent non-metal. Under
normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S
8. Elemental sulfur is a bright yellow
crystalline solid when at room temperature. Chemically, sulfur can react as either an
oxidant or
reducing agent. It oxidizes most
metals and several
nonmetals, including carbon, which leads to its negative charge in most
organosulfur compounds, but it reduces several strong oxidants, such as
oxygen and
fluorine. It is also the lightest element to easily produce stable exceptions to the
octet rule.
In
nature, sulfur can be found as the pure element and as
sulfide and
sulfate minerals. Elemental sulfur crystals are commonly sought after by mineral collectors for their brightly colored
polyhedron shapes. Being abundant in native form, sulfur was known in ancient times, mentioned for its uses in
ancient Greece,
China and
Egypt. Sulfur fumes were used as fumigants, and sulfur-containing medicinal mixtures were used as balms and antiparasitics. Sulfur is referenced in the
Bible as
brimstone in
English, with this name still used in several nonscientific terms.
[2] Sulfur was considered important enough to receive its own
alchemical symbol. It was needed to make the best quality of
black gunpowder, and the bright yellow powder was hypothesized by alchemists to contain some of the properties of gold, which they sought to synthesize from it. In 1777,
Antoine Lavoisier helped convince the scientific community that sulfur was a basic element, rather than a compound.
Elemental sulfur was once extracted from
salt domes where it sometimes occurs in nearly pure form, but this method has been obsolete since the late 20th century. Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from
natural gas and
petroleum. The element's commercial uses are primarily in
fertilizers, because of the relatively high requirement of plants for it, and in the manufacture of
sulfuric acid, a primary industrial chemical. Other well-known uses for the element are in
matches,
insecticides and
fungicides. Many sulfur compounds are odiferous, and the smell of odorized natural gas, skunk scent, grapefruit, and garlic is due to sulfur compounds.
Hydrogen sulfide produced by living organisms imparts the characteristic odor to rotting eggs and other biological processes.
Sulfur is an
essential element for all life, and is widely used in biochemical processes. In metabolic reactions, sulfur compounds serve as both fuels and respiratory (oxygen-replacing) materials for simple organisms. Sulfur in organic form is present in the vitamins
biotin and
thiamine, the latter being named for the Greek word for sulfur. Sulfur is an important part of many enzymes and in antioxidant molecules like
glutathione and
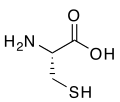
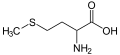
thioredoxin. Organically bonded sulfur is a component of all proteins, as the
amino acids cysteine and
methionine.
Disulfide bonds are largely responsible for the mechanical strength and insolubility of the protein
keratin, found in outer skin, hair, and feathers, and the element contributes to their pungent odor when burned.
Characteristics

When burned, sulfur melts to a blood-red liquid and emits a blue flame which is best observed in the dark.
Physical
Sulfur forms polyatomic molecules with different chemical formulas, with the best-known allotrope being
octasulfur, cyclo-S
8. Octasulfur is a soft, bright-yellow solid with only a faint odor, similar to that of
matches.
[3] It melts at 115.21 °C, boils at 444.6 °C and sublimes easily.
[2] At 95.2 °C, below its melting temperature, cyclo-octasulfur changes from α-octasulfur to the β-
polymorph.
[4] The structure of the S
8 ring is virtually unchanged by this phase change, which affects the intermolecular interactions. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased
viscosity due to the formation of
polymers.
[4] At even higher temperatures, however, the viscosity decreases as depolymerization occurs. Molten sulfur assumes a dark red color above 200 °C. The density of sulfur is about 2 g·cm
−3, depending on the allotrope; all of its stable allotropes are excellent electrical insulators.
Chemical
Sulfur burns with a blue flame concomitant with formation of
sulfur dioxide, notable for its peculiar suffocating odor. Sulfur is insoluble in water but soluble in
carbon disulfide and, to a lesser extent, in other nonpolar organic solvents, such as
benzene and
toluene. The first and the second ionization energies of sulfur are 999.6 and 2252 kJ·mol
−1, respectively. Despite such figures, S
2+ is rare, S
4, 6+ being more common. The fourth and sixth ionization energies are 4556 and 8495.8 kJ·mol
−1, the magnitude of the figures caused by electron transfer between orbitals; these states are only stable with strong oxidants as
fluorine,
oxygen, and
chlorine.
Allotropes

The structure of the cyclooctasulfur molecule, S
8.
Sulfur forms more than 30 solid
allotropes, more than any other element.
[5] Besides S
8, several other rings are known.
[6] Removing one atom from the crown gives S
7, which is more deeply yellow than S
8.
HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S
8, but with S
7 and small amounts of S
6.
[7] Larger rings have been prepared, including S
12 and S
18.
[8][9]
Amorphous or "plastic" sulfur is produced by rapid cooling of molten sulfur—for example, by pouring it into cold water.
X-ray crystallography studies show that the amorphous form may have a
helical structure with eight atoms per turn. The long coiled polymeric molecules cause the brownish substance to be
elastic, and in bulk this form has the feel of crude rubber. This form is
metastable at room temperature and gradually reverts to crystalline molecular allotrope, which is no longer elastic. This process happens within a matter of hours to days, but can be rapidly catalyzed.
Isotopes
Sulfur has 25 known
isotopes, four of which are stable:
32S (95.02%),
33S (0.75%),
34S (4.21%), and
36S (0.02%). Other than
35S, with a
half-life of 87 days and formed in
cosmic ray spallation of
40Ar, the
radioactive isotopes of sulfur have half-lives less than
170 minutes.
When sulfide
minerals are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δS-34 values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The δ
C-13 and δS-34 of coexisting
carbonates and sulfides can be used to determine the
pH and oxygen
fugacity of the ore-bearing fluid during ore formation.
In most
forest ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in
hydrologic studies. Differences in the
natural abundances can be used in systems where there is sufficient variation in the
34S of ecosystem components.
Rocky Mountain lakes thought to be dominated by atmospheric sources of sulfate have been found to have different δ
34S values from lakes believed to be dominated by watershed sources of sulfate.
Natural occurrence

Most of the yellow and orange hues of
Io are due to elemental sulfur and sulfur compounds, produced by active volcanoes.

Native sulfur crystals,
Iran
Sulfur crystals, khanegiran,
Iran
A man carrying sulfur blocks from
Kawah Ijen, a volcano in East Java, Indonesia (photo 2009)
S is created inside massive stars, at a depth where the temperature exceeds 2.5×10
9 K, by the
fusion of one nucleus of silicon plus one nucleus of helium.
[10] As this is part of the
alpha process that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of
meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as
troilite (FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds.
[11] The distinctive colors of
Jupiter's
volcanic moon
Io are attributed to various forms of molten, solid and gaseous sulfur.
[12]
On Earth, elemental sulfur can be found near
hot springs and
volcanic regions in many parts of the world, especially along the
Pacific Ring of Fire; such volcanic deposits are currently mined in
Indonesia,
Chile, and Japan. Such deposits are polycrystalline, with the largest documented single crystal measuring 22×16×11 cm.
[13] Historically,
Sicily was a large source of sulfur in the
Industrial Revolution.
[14]
Significant deposits of elemental sulfur, believed to have been (and are still being) synthesised by
anaerobic bacteria on
sulfate minerals like
gypsum, exist in
salt domes along the coast of the
Gulf of Mexico, and in
evaporites in eastern Europe and western Asia. Native sulfur may be produced by geological processes alone. Fossil-based sulfur deposits from salt domes have until recently been the basis for commercial production in the
United States,
Poland,
Russia,
Turkmenistan, and
Ukraine.
[15] Such sources are now of secondary commercial importance, and most are no longer worked.
Common naturally-occurring sulfur compounds include the
sulfide minerals, such as
pyrite (iron sulfide),
cinnabar (mercury sulfide),
galena (lead sulfide),
sphalerite (zinc sulfide) and
stibnite (antimony sulfide); and the sulfates, such as gypsum (calcium sulfate),
alunite (potassium aluminium sulfate), and
barite (barium sulfate). On Earth, just as upon Jupiter's moon Io, elemental sulfur occurs naturally in volcanic emissions, including emissions from
hydrothermal vents.
Production
Sulfur may be found by itself and historically was usually obtained in this way, while pyrite has been a source of sulfur via sulfuric acid.
[16] The Sicilian process was used in ancient times to obtain sulfur from rocks present in volcanic regions of
Sicily: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them. Then, powdered sulfur was put on top of the deposit and ignited, causing the deposits to melt down the hills. Today's sulfur production is as a side product of other industrial processes such as oil refining; in these processes, sulfur often occurs as undesired or detrimental compounds that are extracted and converted to elemental sulfur. As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of ancient bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by the
Frasch process.
[15] In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the 99.5% pure melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur which required no further purification. However, due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not been employed in a major way anywhere in the world since 2002.
[17][18]
Today, sulfur is produced from petroleum,
natural gas, and related fossil resources, from which it is obtained mainly as
hydrogen sulfide.
Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to
hydrodesulfurization, which cleaves the C–S bonds:
[17][18]
- R-S-R + 2 H2 → 2 RH + H2S
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the
Claus process. This process entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
[17][18]
- 3 O2 + 2 H2S → 2 SO2 + 2 H2O
- SO2 + 2 H2S → 3 S + 2 H2O
Owing to the high sulfur content of the
Athabasca Oil Sands, stockpiles of elemental sulfur from this process now exist throughout
Alberta, Canada.
[19] Another way of storing sulfur is as a
binder for concrete, the resulting product having many desirable properties.
[20] The price of sulfur increased from 2007 to 2008, and decreased thereafter.
[21]
Compounds
Common
oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the
noble gases.
Sulfides
Treatment of sulfur with hydrogen gives
hydrogen sulfide. When dissolved in water, hydrogen sulfide is mildly acidic:
[2]
- H2S
 HS– + H+
HS– + H+
Hydrogen sulfide gas and the dissolved sulfide and hydrosulfide anions are extremely toxic to mammals, due to their inhibition of the oxygen-carrying capacity of hemoglobin and certain
cytochromes in a manner analogous to
cyanide and
azide (see below, under
precautions).
Reduction of elemental sulfur gives
polysulfides, which consist of chains of sulfur atoms terminated with S
– centers:
- 2 Na + S8 → Na2S8
This reaction highlights arguably the single most distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation of these polysulfide anions gives the polysulfanes, H
2S
x where x = 2, 3, and 4.
[22] Ultimately reduction of sulfur gives sulfide salts:
- 16 Na + S8 → 8 Na2S
The interconversion of these species is exploited in the
sodium-sulfur battery. The
radical anion S
3– gives the blue color to the mineral
lapis lazuli.
Elemental sulfur can be oxidized, for example, to give bicyclic S
82+.
Oxides and oxyanions
The principal sulfur oxides are obtained by burning sulfur:
- S + O2 → SO2
- 2 SO2 + O2 → 2 SO3
Other oxides are known, e.g. sulfur monoxide and disulfur mono- and dioxides, but they are unstable.
The sulfur oxides form numerous oxyanions with the formula SO
n2–. Sulfur dioxide and
sulfites (
SO2−
3) are related to the unstable sulfurous acid (H
2SO
3).
Sulfur trioxide and
sulfates (
SO2−
4) are related to
sulfuric acid. Sulfuric acid and SO
3 combine to give oleum, a solution of
pyrosulfuric acid (H
2S
2O
7) in sulfuric acid.
-
Peroxides convert sulfur into unstable such as S
8O, a sulfoxide.
Peroxymonosulfuric acid (H
2SO
5) and
peroxydisulfuric acids (H
2S
2O
8), made from the action of SO
3 on concentrated
H2O2, and
H2SO4 on concentrated H
2O
2 respectively.

The sulfate anion,
SO2−
4 salts (
S2O2−
3), sometimes referred as "hyposulfites", used in photographic fixing (HYPO) and as reducing agents, feature sulfur in two oxidation states.
Sodium dithionite, (
S2O2−
4), contains the more highly reducing dithionite anion.
Sodium dithionate (Na
2S
2O
6) is the first member of the
polythionic acids (H
2S
nO
6), where
n can range from 3 to many.
Halides and oxyhalides
The two main sulfur fluorides are
sulfur hexafluoride, a dense gas used as nonreactive and nontoxic propellant, and
sulfur tetrafluoride, a rarely used organic reagent that is highly toxic.
[23] Their chlorinated analogs are
sulfur dichloride and
sulfur monochloride.
Sulfuryl chloride and
chlorosulfuric acid are derivatives of sulfuric acid;
thionyl chloride (SOCl
2) is a common reagent in
organic synthesis.
[24]
Pnictides
The most important S–N compound is the cage
tetrasulfur tetranitride (S
4N
4). Heating this compound gives
polymeric sulfur nitride ((SN)
x), which has metallic properties even though it does not contain any
metal atoms.
Thiocyanates contain the SCN
− group. Oxidation of thiocyanate gives
thiocyanogen, (SCN)
2 with the connectivity NCS-SCN.
Phosphorus sulfides are numerous, the most important commercially being the cages P
4S
10 and P
4S
3.
[25][26]
Metal sulfides
Many if not most minerals occur as sulfides. The principal ores of copper, zinc, nickel, cobalt, molybdenum and others are sulfides. These materials tend to be dark-colored
semiconductors that are not readily attacked by water or even many acids. They are formed, both geochemically and in the laboratory, by the reaction of hydrogen sulfide with metal salts to form the metal sulfides. The mineral
galena (PbS) was the first demonstrated
semiconductor and found a use as a signal
rectifier in the
cat's whiskers of early
crystal radios. The iron sulfide called
pyrite, the so-called "fool's gold," has the formula FeS
2.
[27] The upgrading of these ores, usually by
roasting, is costly and environmentally hazardous. Sulfur corrodes many metals via the process called
tarnishing.
Organic compounds
- Illustrative organosulfur compounds
Allicin, the active ingredient in garlic

 godiva
godiva












 HS– + H+
HS– + H+